9 Considerations for a Successful LIMS Implementation
In many R&D labs, the moment that exposes weaknesses isn't a major incident, but instead is a routine check. Perhaps a regulator asks for the full record of a sample and the chain of custody cannot be traced to its current location or outcome. Or maybe a project lead requests historical data on an experiment's progress, only to learn it was logged manually and never integrated into the lab's central record-keeping system. Each time this happens, a lab faces delays in advancing research and is exposed to greater vulnerability during audits. What if there were a comprehensive solution that prevented these issues from ever occurring?


A Laboratory Information Management System (LIMS) addresses these problems by creating a unified environment where data, workflows, and sample histories are captured in their entirety. When implemented correctly, it enables leaders to answer questions promptly and with confidence. LIMS are being adopted by modern R&D labs at an increasing rate, with the global LIMS sector expanding from $2.88 billion in 2025 to $5.19 billion by 2030. Organizations are investing at this level because the cost of missing or unreliable data is now too high to ignore.
The challenge for labs is not deciding whether LIMS is needed, but ensuring that it delivers lasting improvements. Let's take a closer look at how to roll out a successful LIMS implementation for your R&D lab.
What is LIMS, and why is it essential in modern labs?
A Laboratory Information Management System lets R&D teams track samples from intake through analysis while keeping all related data and workflows in one place. This minimizes errors and also ensures consistency and audit readiness across the lab.
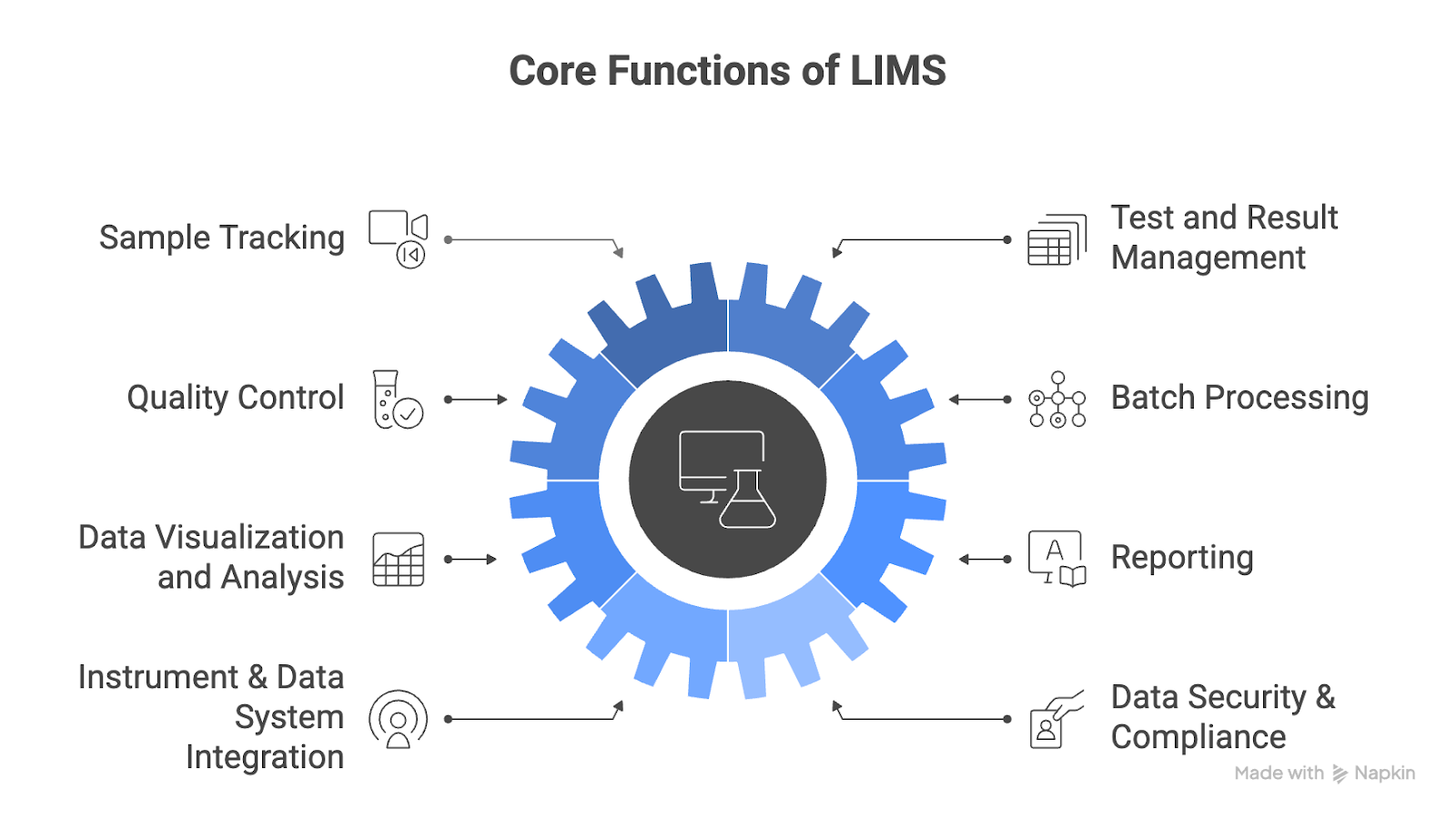
The core functions of a LIMS typically include:
- Sample tracking
- Result management
- Quality control
- Batch processing
- Data visualization and analysis
- Reporting
- Instrument and data system integration
- Data security and compliance (e.g., FDA 21 CFR Part 11, ISO/IEC 17025)
When implemented correctly, a LIMS improves operational efficiency across complex R&D environments. It enables organizations in chemicals, food and beverage, advanced materials, and biopharma to streamline operations, maintain compliance, and safeguard sensitive data.
Benefits of LIMS Implementation
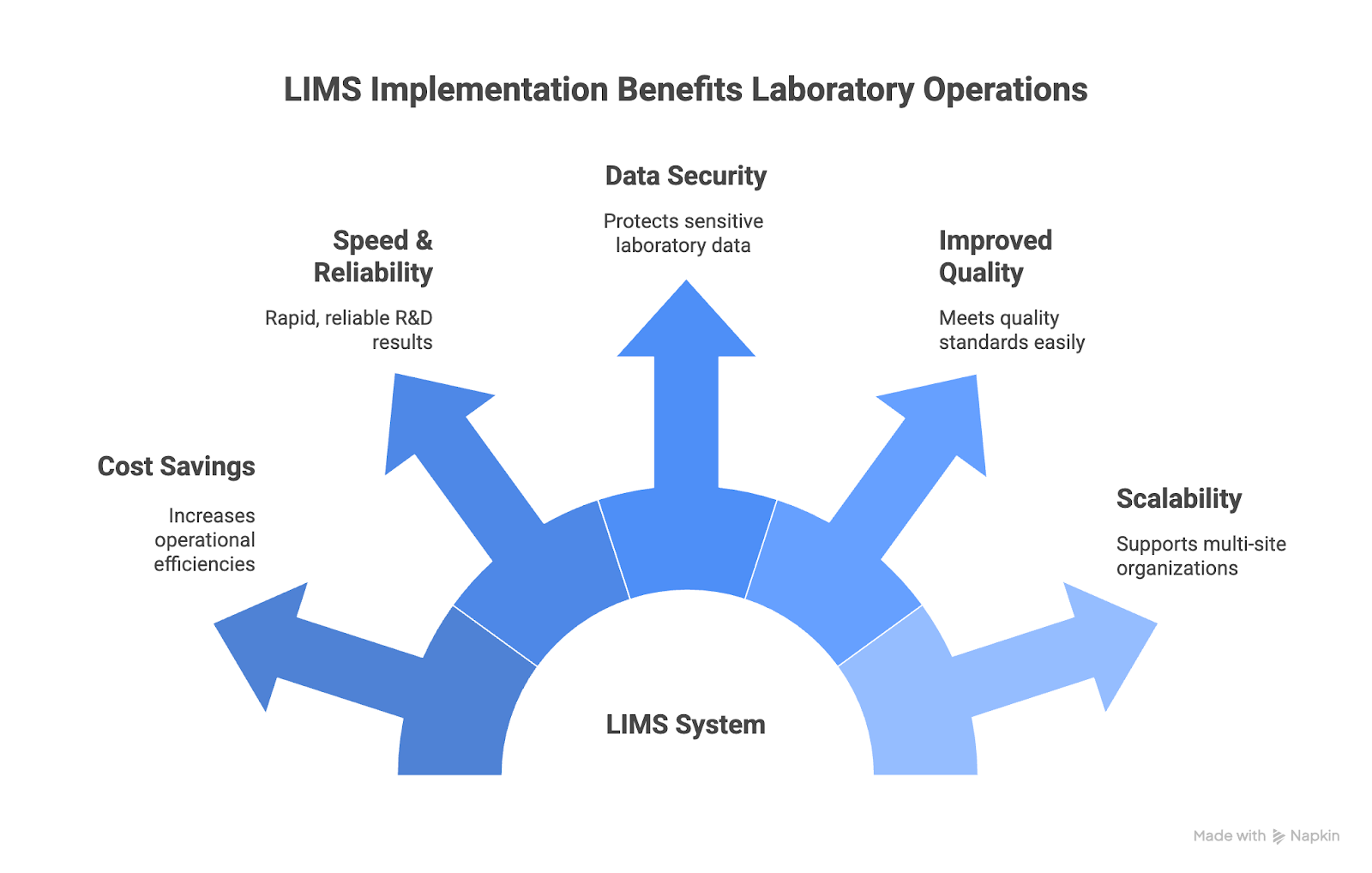
LIMS implementation offers many benefits, including:
- Cost Savings – Standardizing and automating laboratory operations leads to operational efficiencies and increased productivity.
- Increased Speed and Reliability – Automated workflows and data capture allow for the rapid R&D results that pharma, biotech, and other sectors need, while greater standardization and reduced manual errors increase reliability.
- Robust Data Security and Compliance – LIMS helps laboratories protect sensitive data while meeting regulatory requirements such as FDA 21 CFR Part 11 and ISO/IEC 17025. LIMS help maintain complete audit trails and full sample traceability for inspections and quality management, while access control, authentication, and encryption safeguard data integrity and reduce the risk of breaches. Many organizations also strengthen this foundation with external expertise for compliance oversight to ensure data governance extends across all IT systems, not just within the lab.
- Improved Quality – LIMS enables labs to meet quality standards and regulations by providing quality control, sample tracking, unique sample identification, and essential tools for QC processes.
- Scalability – Cloud-based LIMS platforms support multi-site organizations, which enables faster scaling because the same data can be used across multiple geographic locations.
Top 9 Considerations for Successful LIMS Implementation
1. Define Clear Goals and Success Criteria
First, you need to establish clear objectives and success criteria. Ensure all your stakeholders are aligned on exactly which problems the LIMS is solving, which outcomes it needs to support, and how success is measured.
Clear goals transform abstract concepts like "better efficiency" into concrete, measurable outcomes such as "reduce sample processing time by 30%," or "achieve 99.5% data accuracy." These goals also guide major decisions such as system selection and configuration priorities, and determine how stakeholders know whether the implementation project is working.
How to do it:
- Get started by documenting 3-5 specific use cases that the LIMS must solve, such as tracking samples or supporting data export for reporting.
- Detail the measurable performance benchmarks your lab aims to achieve and how these objectives align with business goals, such as reducing costs.
2. Establish a Cross-Functional Implementation Team with Ongoing Ownership
To ensure your LIMS implementation is performed correctly, it's critical to build a team that addresses your core needs and ensures leadership and staff alignment. The implementation team should include stakeholders from different functions and bridge the gap between technical requirements, operational realities, and business/leadership needs.
It may include roles such as laboratory management, QA/QC, IT, regulatory compliance, and leadership. This team should be in it for the long haul, beyond going live to own configuration, validation, training, change management, and support. Their goal is to ensure the LIMS is properly implemented and is actively being used the right way.
How to do it:
- Assign formal implementation team roles as the project kicks off.
- To ensure a smooth transition from LIMS implementation to LIMS adoption, plan responsibilities for when the LIMS goes live, and define post-launch responsibilities.
3. Engage Stakeholders and Prepare for Change Early
Change resistance is one of the most common challenges in LIMS implementation. It often stems from end-users, such as scientists, lab managers, and QA personnel, who weren't involved in the decision-making process. However, early stakeholder engagement transforms potential resistors into champions, which significantly improves adoption rates and long-term success.
LIMS implementation fundamentally changes how laboratory professionals perform their daily work. While using the system correctly will ultimately increase lab productivity, user rejection could block it. That makes it critical to secure stakeholder buy-in from the outset so that your lab staff don't feel like they were handed a new system without the necessary context, training, or input.
How to do it:
- Building a communication and change management plan into the rollout.
- Run stakeholder interviews, demos, and pilot workflows early so that lab staff have a chance to shape how the LIMS platform operates and what their day-to-day will look like.

4. Map and Standardize Lab Workflows Before Configuration
LIMS platforms require structured and consistent processes to function effectively. It's crucial to document the current ("as-is") workflows first, then decide on any changes needed in the future-state ("to-be") processes before configuring the system. This step is crucial because LIMS enforces structure, but doesn't fix poor or inconsistent processes.
How to do it:
- Review all standard operating procedures (SOPs), sample flow diagrams, and exception-handling documentation as pre-configuration inputs.
- Detail current processes and retain what works well. Update what doesn't and improve it by incorporating LIMS capabilities.
5. Treat Data Migration as a Dedicated Workstream
Data migration requires dedicated project management attention because poor data quality undermines trust in the new system from the outset. Legacy data often contains inconsistencies, duplicates, and formatting issues that become magnified in structured LIMS environments. Proper data migration requires data cleanup, field mapping, legacy data structuring, and validation protocols as an integral part of the transition.
How to do it:
- Build a data inventory.
- Categorize your data sources.
- Validate migrated records in a test environment with lab users.
6. Plan System Integrations from the Start
A LIMS delivers its full value only when it connects smoothly with the other systems your lab relies on. Integration planning should begin early in the project so that data exchange is designed into the system from the outset.
Without integration, your LIMS risks becoming a silo that forces duplicate data entry and breaks workflows. Link the LIMS to instruments, electronic lab notebooks (ELNs), inventory systems, and enterprise platforms like ERP or CRMs. This linkage ensures accurate data flows, reduces manual effort, and extends its usefulness across the organization. In practice, this is enabled through middleware and APIs, which act as bridges between systems—allowing secure, automated data exchange without manual re-entry or risk of errors.
How to do it:
- Identify the systems and instruments that must exchange data with the LIMS.
- Define integration requirements, including data formats and workflows.
- Build these requirements into the project scope.
- Test integrations to confirm accuracy and reliability before rollout.

7. Configure the System Based on Real Lab Needs
LIMS platforms offer extensive configuration options, but their default settings rarely reflect the actual workflow of real laboratories. Laboratories should configure the LIMS to reflect the language, workflows, user permissions, and exception patterns that reflect how their lab actually operates. This enhanced usability increases the likelihood that end users will adopt the platform in their day-to-day work.
How to do it:
- Define your lab's needs in requirements workshops.
- Design LIMS configurations in collaboration with lab users to ensure optimal functionality.
- Document all requirements and changes for validation and support.
8. Roll Out LIMS in Phases
Phased rollouts significantly improve LIMS implementation by allowing organizations to identify and resolve issues with smaller user groups before deploying to the whole organization. A phased approach could begin with small pilot groups and user acceptance testing (UAT) to verify that the software functions properly in real-world scenarios.
At that point, the rollout can expand to larger groups and incorporate training programs to ensure everyone knows how to use the LIMS. Incorporating feedback sessions allows your lab staff to provide input on how the platform is working and raise any issues. The real implementation ends only after users are trained, workflows are stable, and the LIMS is in day-to-day use.
How to do it:
- Require role-based LIMS training for staff and stakeholders.
- Establish feedback channels to ensure that lab staff have a means to express ideas for changes or suggested fixes.
- Monitor adoption metrics and refine the workflows and other configurations based on usage.
9. Implement a LIMS That Combines Process Control with R&D Insight
A standard LIMS covers the essentials: sample tracking, workflow control, audit trails, and compliance. But for materials-driven R&D, that is only part of what's required. Researchers also need tools for experiment documentation, data reuse, visualization, and collaboration across sites.
MaterialsZone brings these elements together in a single platform. By combining LIMS and ELN functionality with advanced materials informatics, it provides both the process control labs needed for compliance and the insight they need to accelerate R&D. The result is an environment where routine operations and exploratory research reinforce each other, shortening development cycles and improving decision-making.
How to do it:
- Choose a platform that combines LIMS and ELN with advanced materials informatics.
- Ensure it supports experiment documentation, data reuse, visualization, and collaboration.
- The platform should include integrated analytics and AI that guide R&D and help reduce the number of required iterations.
- Select a system that unifies compliance-focused LIMS functionality with the tools researchers need for data analysis, experiment tracking, and collaboration.
Drive R&D Value Through Successful LIMS Implementation
LIMS implementation is one of the most effective steps an R&D laboratory can take to improve the reliability and efficiency of its operations. When carried out correctly, it provides lab teams with a single framework that supports accurate data capture, secure storage, and smoother workflows. For R&D labs, the outcome is faster progress and a stronger foundation for compliance.
MaterialsZone extends these benefits by combining LIMS functionality with advanced materials informatics. This integration creates a single environment where routine testing and exploratory research can be managed together, with analytics and AI providing the insight needed to shorten development cycles and accelerate decision-making.
Request a MaterialsZone demo today to discover how it goes beyond LIMS to help you bring better products to the market faster.




