What is Total Dissolved Solids (TDS)? Definition, Guides, and Examples
Water is used in materials R&D for cleaning, dilution, synthesis, and as a raw material. Most labs treat it as stable because it's filtered or comes from a regulated source; however, this assumption doesn't always hold.
.png)

Consider this common laboratory scenario: Researchers begin to see inconsistencies in an experiment's results. Reaction yields drop, particle sizes shift, and the test's reproducibility declines. After reviewing their procedures and raw materials, the issues are traced back to the lab's water—a recent spike in Total Dissolved Solids (TDS) caused by seasonal runoff was affecting solubility and pH control, which undermined an otherwise stable process.
Lab teams routinely trust that their water supply is "good enough." This is especially true in the U.S., where 9 out of 10 people get their tap water from a public water system, and utilities are required to meet Environmental Protection Agency (EPA) standards for over 90 contaminants. But even with this regulatory oversight, the invisible world of dissolved solids can undermine the most advanced R&D or manufacturing workflows. Because the EPA's secondary limit for TDS is 500 mg/L, which is set for taste and clarity rather than scientific control, labs relying on EPA-compliant water can still face sudden variability. These water quality fluctuations can derail critical formulations, introduce untraceable process drift, and damage sensitive equipment.
In today's precision-driven R&D environment, it's no longer enough to assume water is a "controlled variable." Systematically tracking and controlling TDS is essential for process integrity and product quality. Here's what you need to know about total dissolved solids and how they can affect your R&D lab results.
What is Total Dissolved Solids (TDS), and Why Is It Important?
Total Dissolved Solids (TDS) represents the total concentration of dissolved substances in water, measured in parts per million (ppm) or milligrams per liter (mg/L). Chemically, these include inorganic salts such as sodium, calcium, magnesium, chloride, sulfate, and bicarbonate, along with trace organics, metals, and other small molecules that remain in solution after standard filtration. In most contexts, this refers to filtration through a 2-micron pore size.
Total dissolved solids in water originate from natural sources, such as minerals in groundwater or leaching from riverbeds, as well as from urban and industrial runoff and water treatment chemicals. Water distribution infrastructure also contributes to TDS. Aging pipes can leach metals into the supply, and water softeners increase sodium concentrations.

In the United States, public utilities monitor TDS as part of general water quality assessment. However, it is not regulated as a primary contaminant. The EPA's secondary maximum contaminant level for TDS is 500 mg/L, which is based on aesthetic concerns such as taste, clarity, and odor rather than health effects.
Concentrations of TDS can vary due to seasonal changes, changes in source water, or infrastructure maintenance. These fluctuations can introduce unwanted variability into lab procedures that depend on consistent water quality.
The Relevance of TDS to Drinking Water and Laboratories
In public water systems, water with high TDS may taste salty or metallic and can appear cloudy, but it is still considered safe for consumption. Because the standard is aesthetic, it is not enforceable, and utilities are not required to reduce TDS unless there are customer complaints.
In industrial, pharmaceutical, and research environments, the requirements are stricter. TDS levels that are acceptable for drinking can interfere with sensitive processes. Many lab workflows depend on low and stable TDS concentrations to maintain consistency.
High or variable TDS can:
- Shift chemical solubility and interfere with reaction kinetics
- Disrupt buffer systems and pH control, which are essential for synthesis and analysis
- Promote scaling and fouling in equipment that uses or circulates water, such as viscometers.
- Introduce variability that affects batch consistency and undermines reproducibility.
Because TDS is not routinely tracked in many labs, these effects often go unrecognized until they affect product quality or experimental outcomes.
Total Dissolved Solids (TDS) vs. Total Suspended Solids (TSS)
TDS and TSS are both used to assess water quality, but they measure different categories of material.
- Total Dissolved Solids (TDS) refers to substances that are fully dissolved in water, such as ions and small molecules. These cannot be seen and will pass through fine filtration.
- Total Suspended Solids (TSS) refers to larger particles that remain physically suspended in water, including silt, sand, and organic debris. These can usually be removed through standard sediment filtration, such as mesh filters or cartridge-based systems.
This distinction is vital in lab environments. TSS is typically eliminated early in the water treatment process, meaning it poses little risk to experimental processes. TDS, however, remains present unless the water is further treated using methods like reverse osmosis, deionization, or distillation. Because TDS can influence solubility, pH, and reaction conditions, it is necessary to track it separately.
For example, after a snowstorm, a municipal treatment plant may report elevated TDS due to road salt runoff. Sodium and chloride ions dissolve completely and enter the water supply. At the same time, sand and debris increase the TSS, but these are filtered out before the water reaches end users.
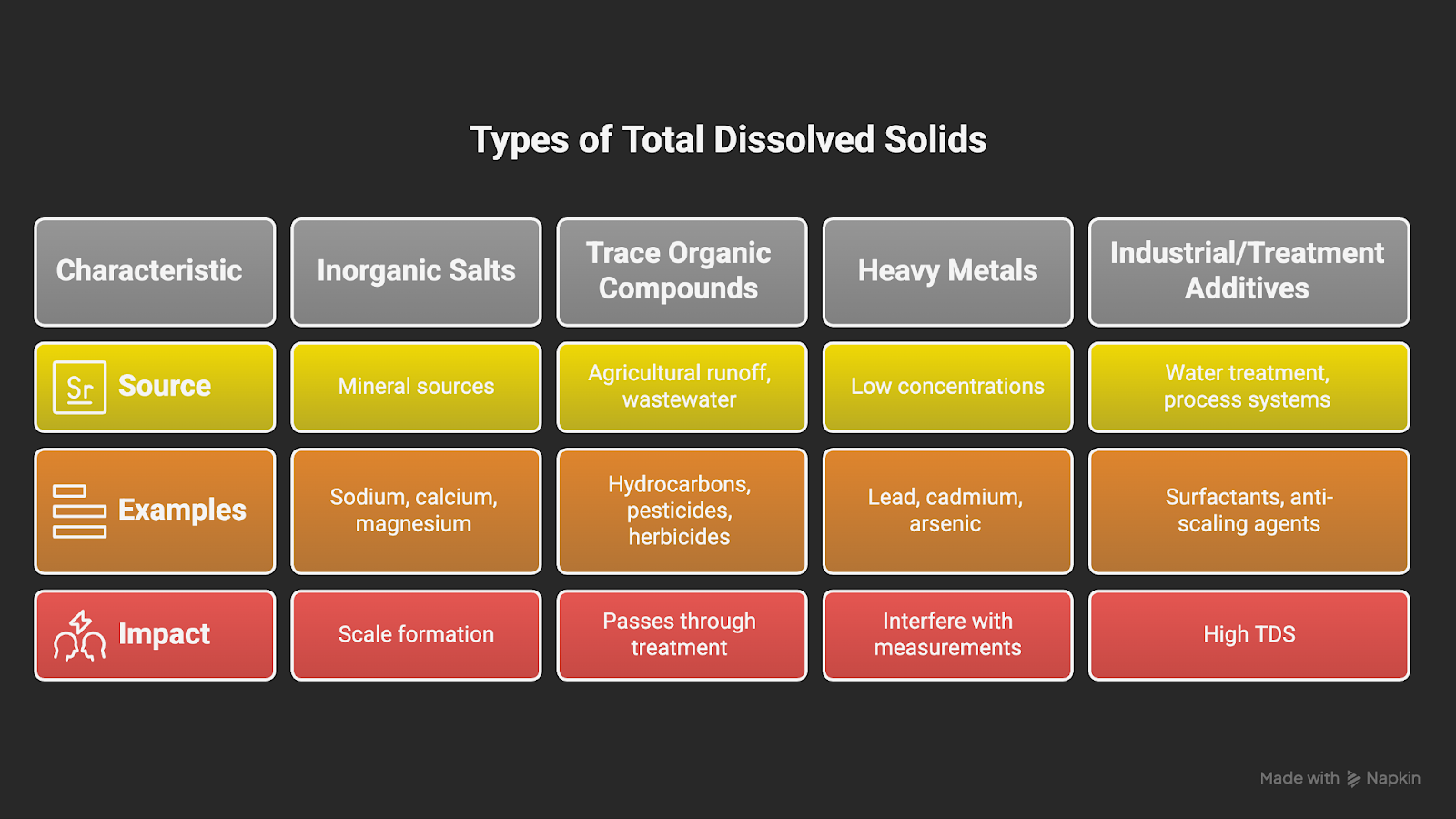
4 Types of Total Dissolved Solids (TDS)
In scientific and industrial settings, TDS substances are typically grouped into these categories:
1. Inorganic Salts
- Inorganic salts are the most common contributors to TDS and include dissolved ions from mineral sources.
- Cations include sodium (Na⁺), calcium (Ca²⁺), magnesium (Mg²⁺), and potassium (K⁺).
- Anions include chloride (Cl⁻), sulfate (SO₄²⁻), nitrate (NO₃⁻), and bicarbonate (HCO₃⁻).
- Example: Calcium and magnesium contribute to water hardness and are often responsible for scale formation in heat exchangers, autoclaves, and lab glassware.
2. Trace Organic Compounds
- This group includes hydrocarbons, pesticides, herbicides, industrial solvents, and low-molecular-weight organic acids.
- These compounds may originate from agricultural runoff, treated wastewater, or decaying organic matter.
- Example: Surface water impacted by agricultural activity may contain trace levels of herbicides that pass through standard municipal treatment and contribute to the TDS reading.
3. Heavy Metals
- Metals such as lead, cadmium, arsenic, and mercury can appear in low concentrations but carry high regulatory importance.
- These elements are not only toxic at parts-per-billion levels but can also interfere with sensitive lab measurements, equipment surfaces, and product purity.
- Example: A pharmaceutical lab using municipal water for initial cleaning steps detected trace cadmium in rinse validation tests. While the concentration was within safe limits for drinking water, it exceeded internal process specs and triggered a review.
4. Industrial and Treatment Additives
- TDS can also include residues from surfactants, anti-scaling agents, phosphates, and cleaning chemicals used in water treatment or process systems.
- Example: A wastewater management solution might report high TDS dominated by sodium chloride from softeners and deicing runoff, with additional contributions from fertilizer nitrates, detergent phosphates, and trace organic contaminants.
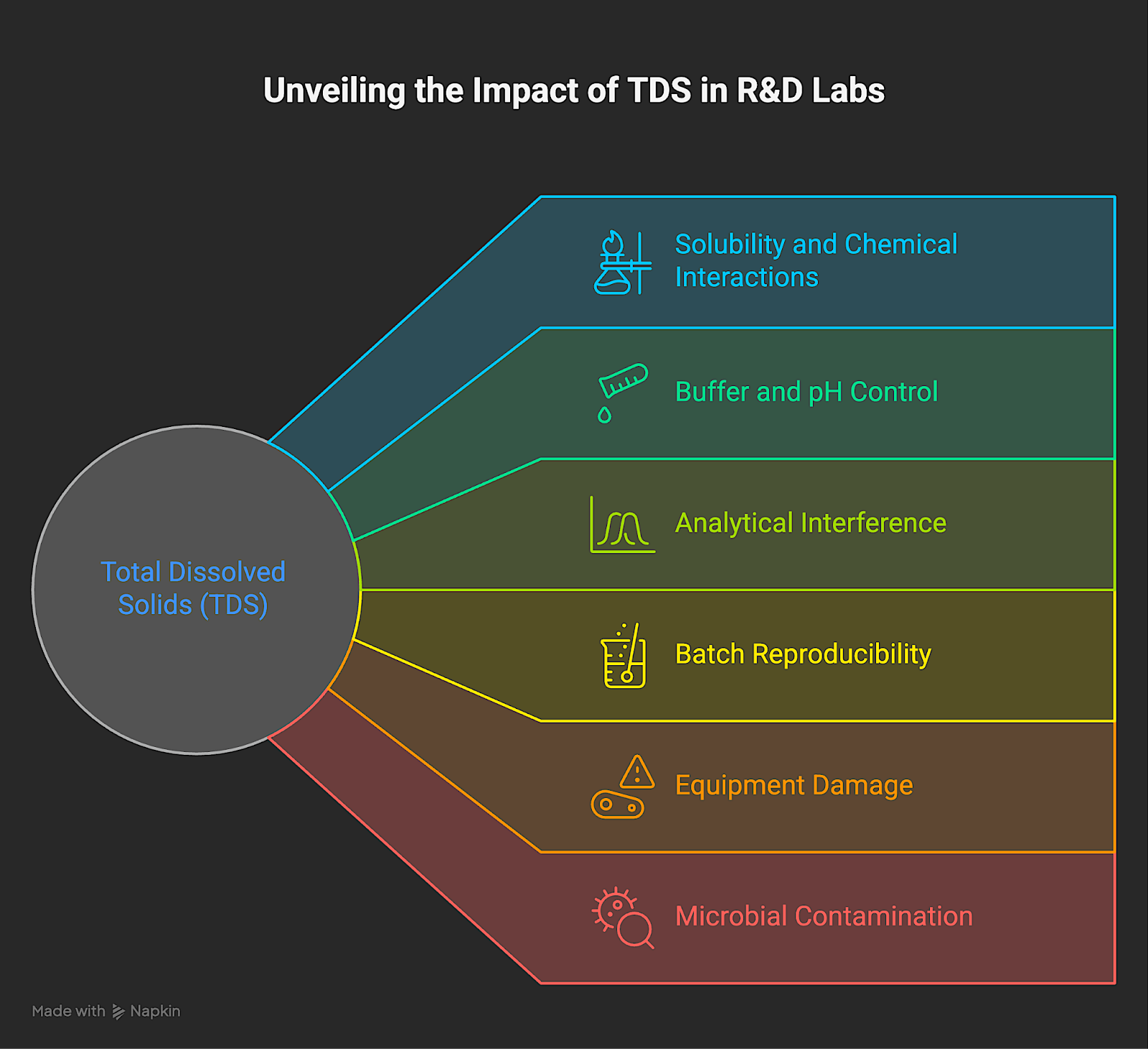
Why Total Dissolved Solids in Water Matter in R&D Labs
For process-driven, data-centric labs, untracked TDS is a recipe for unreliable science. Even small and rapid changes in TDS are operationally disruptive, especially as processes become more sensitive. Here's how:
Solubility and Chemical Interactions
Dissolved ions change the ionic strength of solutions, directly affecting solubility, reactivity, and stability. For example, in nanomaterial synthesis, small TDS fluctuations can alter particle size and distribution, which in turn impact product performance.
Buffer and pH Control
Most biological and chemical processes depend on stable pH buffers. Ions from TDS can consume or alter buffer components, causing an unexpected shift in pH. When formulating pharmaceuticals, protein purification batches are known to fail when buffer pH drifts by 0.2 units due to overlooked sodium influx from water supply maintenance.
Analytical Interference
Elevated or inconsistent TDS can affect the performance of analytical methods by altering ionic strength, shifting baselines, or introducing background signals. These effects may not show up in early-stage tests or demos, but they can compromise accuracy and reproducibility in full-scale validation.
Batch Reproducibility Across Time or Sites
When TDS isn't tracked, water used for rinsing, dilution, or process feeds introduces uncontrolled variability. Two facilities using the same formulation will produce different outcomes because one city's tap water has 250 mg/L TDS and another's has 40 mg/L, despite both meeting EPA standards.
Equipment Damage and Process Downtime
High TDS causes scaling and fouling in heat exchangers, autoclaves, and reactors, resulting in increased cleaning cycles, higher maintenance costs, and premature equipment failure. For example, a reverse osmosis system in a pharma plant may need a costly membrane replacement after unnoticed TDS creep damages its filter.
Microbial Contamination
Organic total dissolved solids can act as a nutrient source for bacteria and fungi. These contaminants create risk in systems that rely on water purity, particularly in sterile production or sensitive analytical environments. If unchecked, microbial growth may lead to biofilm formation, sample contamination, or other process disruptions that compromise reliability.
How to Monitor & Control Total Dissolved Solids in the Lab
Monitoring the total dissolved solids in water is helpful in detecting changes in water quality that could affect lab workflows, especially when purity or consistency is critical. This proactive, system-level discipline involves:
1. Purification System Selection
Select water systems such as reverse osmosis (RO), deionization (DI), or distillation based on your lab requirements. RO removes 95–99% of TDS, but only if membranes and pre-filters are properly maintained.
2. Maintenance and Calibration
Regularly inspect and service all water purification components, including pre-filters, reverse osmosis membranes, deionization resins, and storage tanks, to ensure optimal performance. TDS creep is often first detected after missed maintenance or following municipal water changes that affect source quality.
3. Routine TDS Monitoring
Measure TDS at critical control points in the water system, including the incoming supply, pre-process stages, post-process outlets, and high-risk contact points such as rinse lines or dilution stations. Use hand-held meters for routine checks and log all readings to maintain traceability.
4. Data Integration and Traceability
Treat TDS data like pH or temperature. Log it with every experiment, batch, or cleaning cycle. An advanced materials informatics platform, such as MaterialsZone, helps automate data integration and traceability by recording TDS readings alongside all other experimental data, which enables ease of correlation, rapid troubleshooting, and long-term pattern analysis.

5. Actionable Controls and Documentation
Set thresholds for TDS based on the sensitivity of the process or experiment. If the value exceeds limits, halt processes, inspect all your water systems, and document corrective actions. Include TDS in all SOPs and batch records for full auditability.
A Guide to Measuring Total Dissolved Solids in Water
Total dissolved solids are measured to understand the total concentration of dissolved material in water. The value is expressed in milligrams per liter (mg/L) or parts per million (ppm). While this measurement indicates how much is dissolved, it does not identify the specific substances present.
Two Measurement Methods for Total Dissolved Solids in Water
1. Gravimetric Method (Lab-Based)
This method involves evaporating a known volume of water and weighing the resulting dry residue. It provides a direct measurement of the total dissolved solids in water, and is used when accuracy is more important than speed. Because it requires time, equipment, and stable conditions, it is typically reserved for calibration or regulatory testing.
2. Conductivity-Based Meters (TDS Meters)
Most labs and process environments use conductivity meters for routine TDS measurement. Dissolved ions in water conduct electricity, so electrical conductivity (EC) is measured and converted into a TDS estimate using a conversion factor. The factor varies depending on the ionic profile but usually falls between 0.5 and 0.7. This method is fast, inexpensive, and suitable for continuous or spot-check monitoring.
Interpreting TDS Values in R&D Contexts
The interpretation of TDS values depends on the application. The same number might be acceptable in one context and problematic in another. Here are the typical TDS ranges in ppm, and their relevance in lab settings:
- <1 ppm: Ultrapure water, suitable for semiconductor work, pharmaceutical synthesis, or critical analytics
- 1–50 ppm: High-quality deionized or reverse osmosis water, standard in research labs and cleanroom prep
- 50–250 ppm: Tap or filtered water, usable for non-critical rinsing or cleaning, but not ideal for precision work
- >250 ppm: Outside the range for most R&D and manufacturing use; may cause interference or variability
There is no universal cutoff for what is considered "acceptable" TDS. What matters is that the value is stable and appropriate for the task. Labs should treat TDS like pH or temperature, which is a variable to measure, control, and document. Sudden changes in TDS values are often the first sign that something in the system needs attention.
Make Water Quality a Controlled Variable
TDS is often overlooked in materials R&D, but it can impact key parameters such as solubility, pH control, and process stability. Water that meets regulatory standards may still introduce variability when its composition changes, especially in labs that use treated or recycled water. When these shifts go unmeasured, teams are left reacting to failures without enough information to prevent them.
MaterialsZone enables R&D teams to capture TDS measurements as part of their experimental data. By integrating water quality with formulation, synthesis, and testing records, the platform links outcomes to the conditions present during preparation and processing. It helps teams reduce untracked variability and avoid repeating failed work due to uncontrolled inputs.
Request a demo today to see how MaterialsZone can help your team monitor and manage total dissolved solids in water as part of a reproducible, data-driven R&D workflow.




