The 12 Principles of Green Chemistry Explained
Traditional chemical development is increasingly misaligned with modern environmental and safety expectations. The costs of an approach that optimizes performance first and deals with waste and toxicity as an afterthought have become all too apparent, and demand is surging for safer-by-design chemical products that minimize waste and environmental impact.


High-profile calls for the urgent implementation of sustainable green chemistry have made it clear that the status quo is no longer acceptable as the default way to innovate. Where does this leave chemical R&D teams?
Recognition of the fact that green chemistry is the alternative that builds in safety and sustainability from the very beginning is driving the growth of a market that is expected to reach nearly $230 billion by 2030. It’s a practical approach that prevents waste and reduces hazards at the reaction, solvent, feedtock, and process level, built upon a shared framework of twelve principles that inform concrete R&D choices.
For R&D leaders, the question is whether teams can apply the twelve principles consistently across diverse projects. Implementing them at scale requires data, traceability, and predictive insight, which is why support from the right materials R&D system is critical. Before making this leap, you’ll want to get familiar with the twelve principles of green chemistry and how to implement them effectively, so let’s dig in.
What is Green Chemistry?
Green chemistry is an approach to chemical products and processes that seeks to minimize waste and harmful side effects wherever possible. In 1998, the publication of Green Chemistry, Theory, and Practice by Paul Anastas and John Warner formalized this idea around a set of twelve principles.
Scientific and industrial researchers can follow the guidelines of green chemistry to develop safer, cleaner chemicals that pose less hazard to the environment in which they are produced and the people who use them.
3 Criteria of a Commercially Successful Green Chemistry Product
Green chemistry takes the entire lifecycle of the chemical into account, from sourcing to disposal, but for viability in the commercial market, it isn’t enough for green chemicals to be more eco-friendly than their competitors. Successful products developed using the twelve principles of green chemistry generally meet three conditions:
- The intrinsic hazard profile is reduced or eliminated compared to conventional alternatives, meaning that the product presents lower risk to human health or the environment than existing options.
- The product performs at least as well as the material it replaces. Sustainability improvements cannot compromise the technical requirements of the intended application.
- The product is economically viable in real-world markets. It must be feasible to manufacture and adopt at scale within existing commercial constraints.
Without all three, even well-intentioned innovations struggle to move beyond the lab and into widespread use.
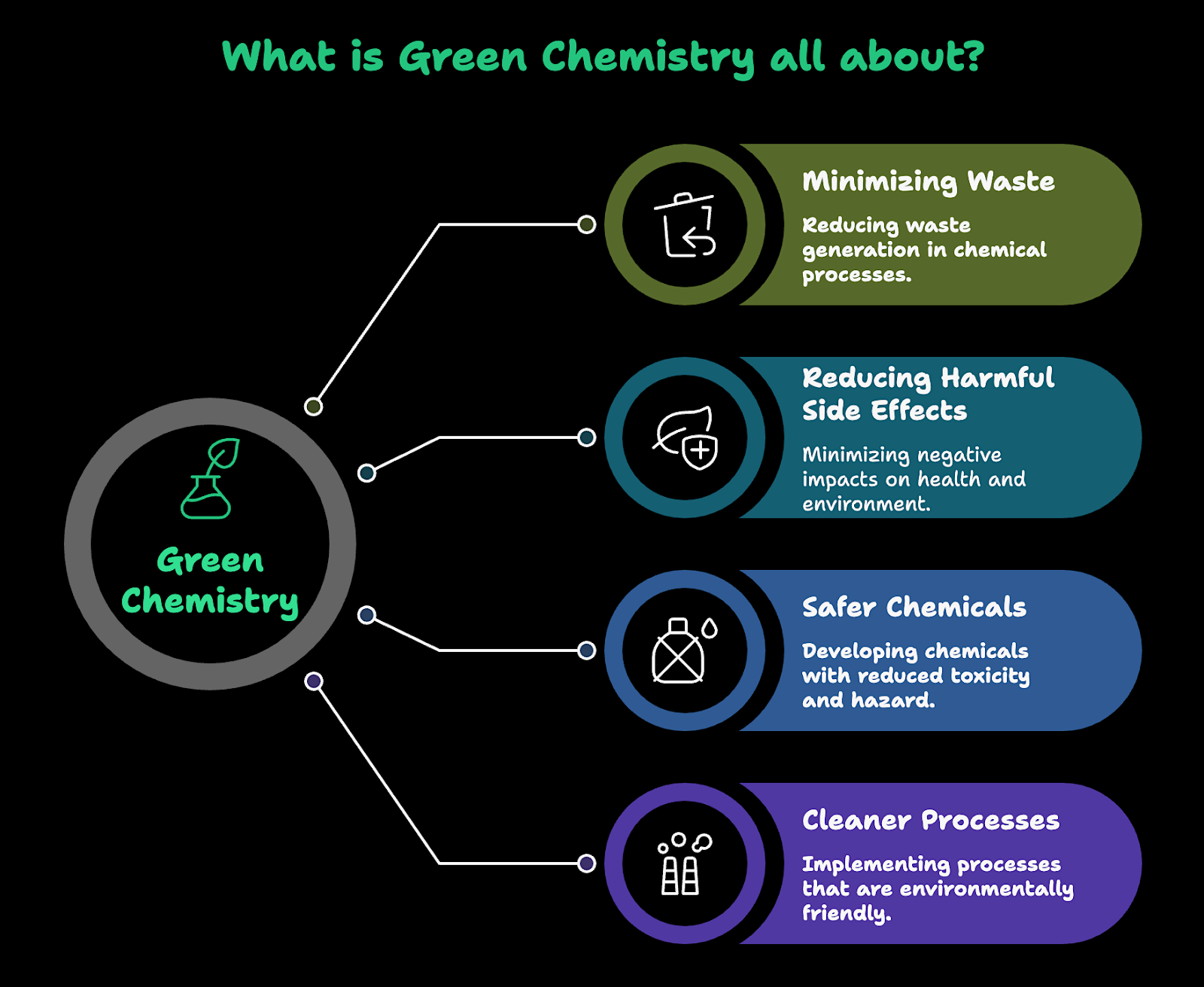
The 12 Principles of Green Chemistry: 5 Key Benefits
Green chemistry that meets the standards of commercial viability is another example of when doing the environmentally responsible thing is also beneficial to a company’s bottom line. Here are a few of the key benefits that the implementation of its twelve principles can provide:
- Greater robustness and efficiency of chemical products and processes
- Faster, more predictable R&D cycles, especially when supported by an AI-driven materials informatics platform
- Waste prevention at the source instead of costly after-the-fact cleanup
- Safer chemistry by design that reduces accidents and mitigation costs throughout the entire product lifecycle
- Longer timelines for the viability of products and portfolios
What are the 12 Principles of Green Chemistry?
These twelve principles provide a structured framework for designing safer, more sustainable chemical products and processes:
1. Prevention
The first principle states that it is more effective to prevent waste than to treat waste, or to clean it up after it has been created, and this serves as a north star for the whole approach. This prevention-first mindset is consistent with environmental management frameworks such as ISO 14001, which formalize systematic identification and reduction of environmental impacts (including pollution-prevention commitments) at the organizational level.
Preventative design is a key concern at every stage in the process, from developing chemicals that require fewer purification steps and generate fewer byproducts, to optimized trial environments that minimize failures and material waste. This emphasis on eliminating inefficiencies reflects the broader logic behind Lean principles.
2. Atom Economy
In chemistry, waste occurs at the atomic level. The principle of “atom economy” holds that syntheses should be designed to incorporate the maximum proportion of reactants into the final product. By dividing the weight of atoms utilized by the weight of all reactants, atom economy metrics can be tracked, and teams can compare data from historical reaction pathways to find more efficient routes.
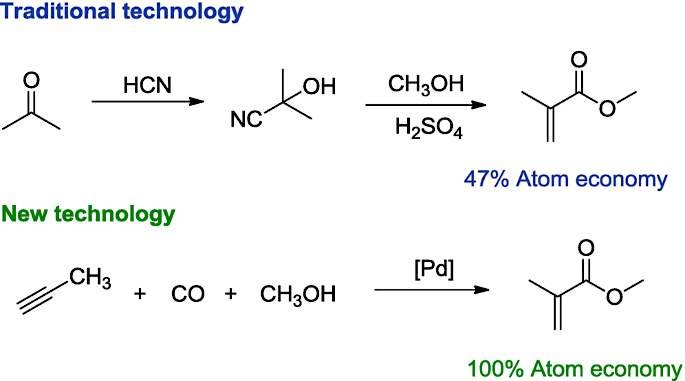
3. Less Hazardous Chemical Syntheses
As much as possible, synthetic methods should use and generate minimally toxic substances. Hazard reduction begins with routing decisions made early in development, such as avoiding extreme reagents and reaction conditions.
Cheap, proven substances are often used as raw materials, and the challenge in green chemistry is in finding economically viable substitutes that don’t require or produce substances that are harmful to human health or the environment.
4. Design Safer Chemicals
The overall goal for green chemical design is to create products that are fully effective for their intended purpose while minimizing toxicity. When green chemicals are designed to replace existing products, they should perform at the same or better level of functionality, but use or generate fewer toxins.
This principle shifts the focus of safety considerations to the molecular and formulation design stage, rather than leaving it all for downstream controls. Factoring performance data into this stage can help teams make more informed tradeoffs. Clear data governance ensures that toxicity and performance metrics are evaluated consistently across formulations.
5. Safer Solvents and Auxiliaries
Solvents, separation agents, processing aids, and other auxiliaries should be eliminated wherever possible, or at a minimum, made from non-hazardous substances. These materials account for a large share of waste and exposure risk, and the successful implementation of this principle can result in significant harm reduction.
Solutions like MaterialsZone’s Materials Knowledge Center centralize SDS data and solvent performance history, which makes it possible to evaluate safer alternatives earlier in the process.
6. Design for Energy Efficiency
Chemicals with special energy requirements are always going to use more resources and have a greater impact on the environment. Green chemistry would have these materials phased out wherever feasible, replaced with syntheses that can be conducted under ambient temperature and pressure.
Labs can utilize MaterialZone’s Visual Analyzer to identify the temporal and physical conditions that drive energy-intensive steps and help find areas where efficiency can be improved.
7. Use of Renewable Feedstocks
The raw materials used for chemical production, or “feedstocks,” should be renewable (like the byproducts of farming and other industrial operations), not depletable (like gas and mineral resources that have to be extracted from deposits in the earth). While it can be challenging to implement in every case, this principle is key to long-term resource sustainability.
Using an advanced materials informatics solution to track raw material usage across projects supports the evaluation and substitution of renewable feedstocks, helping you uncover new opportunities to make these changes.
8. Reduce Derivatives
Chemical derivatives should be avoided, as these use additional reagents and lead to increased waste production. These include blocking and protecting groups, and other temporary modifiers. Derivatization can help steer reactions in the direction you want, but it adds time and complexity to the process, with potential for preventable waste generation at every step.
9. Catalysis (Not Stoichiometric Reagents)
Green chemistry favors catalytic reagents over stoichiometric ones. The reason for this is that catalysts improve selectivity and material utilization while simultaneously reducing waste, because they are effective in small quantities and can affect a single reaction many times over.
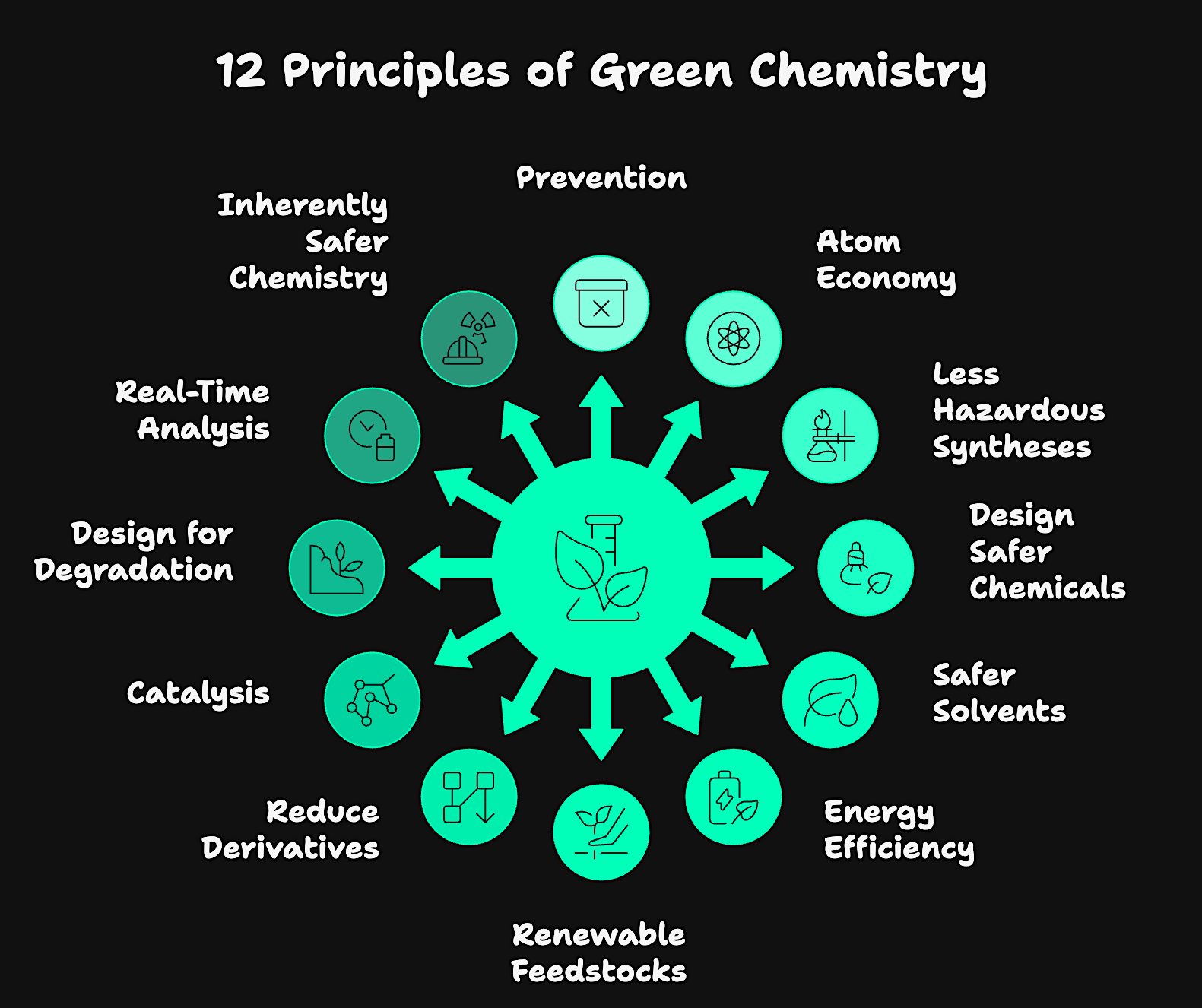
10. Design for Degradation
When green chemicals break down, they should leave behind only non-persistent, harmless substances. Designing for degradation emphasizes the need to address environmental impact far beyond the functional lifetime of the product.
11. Real-Time Analysis for Pollution Prevention
To prevent pollution at the source, develop analytical methodologies that enable you to monitor and control processes in real time, prior to the formation of hazardous off-spec materials. Early detection will help you avoid large-scale waste and reprocessing, which can be supported by employing an AI-guided R&D platform. Using machine learning models and advanced analytics, these tools can identify deviations before scale-up or batch failure.
12. Inherently Safer Chemistry for Accident Prevention
The substances used in a chemical process, and the forms they take, should be designed to prevent accidents such as explosions, fires, and other releases. The priority for this principle is eliminating the potential for hazard rather than controlling it after the fact. From lab to production, consistency can be improved and human error mitigated by introducing standardized procedures, version control, and collaboration tools.
Use Smarter Tools to Make Greener Chemicals
The health and environmental challenges of the future can be met with a sustainable approach to chemical development based on safety and waste reduction. Implementing the twelve principles of green chemistry translates this process into measurable design decisions, but the realities of the development process can quickly put ideals to the test.
One effective way to implement these principles and get them to stick is to use an advanced materials informatics solution that is inherently aligned with the goals of green chemistry. MaterialsZone’s AI-guided R&D platform optimizes your development cycles, helping you achieve fewer iterations and reduced waste.
Connect with MaterialsZone today to see how the platform provides your organization with the tools and insights that lead to cleaner, safer chemical products.



