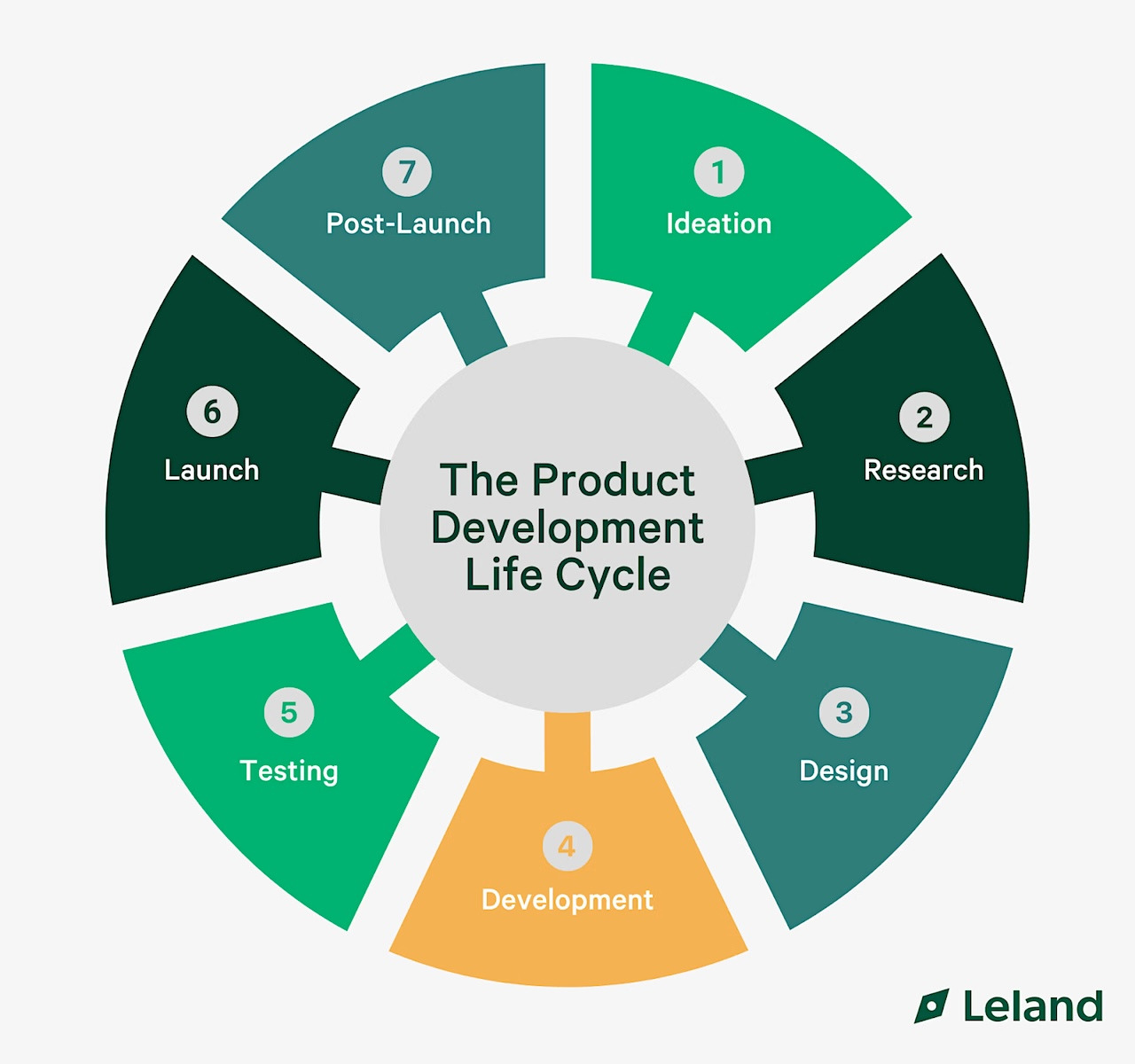
7 Stages to the Product Development Life Cycle
In recent years, product development has undergone significant changes. R&D teams are moving away from rigid stage-gate systems toward more adaptive, data-supported work. However, many organizations still rely on development practices that split experiments, process data, and operational information across separate systems. This fragmentation makes it hard to understand how earlier work connects to current decisions and increases the likelihood of late-stage development setbacks.


A better way to manage development work is to anchor it in the product development life cycle (PDLC). A structured process that guides how ideas move from early exploration into validated products, the PDLC allows teams to evaluate progress with evidence rather than intuition. Treating the PDLC as a dynamic, evidence-based system and investing in digital product development can raise efficiency by up to 19% and cut time-to-market by 17%.
For materials-based industries, where each experiment requires significant time and resources, a modern PDLC is essential for accelerating processes without compromising on quality. From day one, it also helps improve predictability while embedding sustainability and compliance. Let’s unpack what the product development life cycle is all about and explore the seven stages that support consistent, data-driven development.
The Product Development Life Cycle (PDLC): What is it, and why is it important?
The Product Development Life Cycle (PDLC) is the end-to-end process of turning an idea into a market-ready product, and then improving it over time. Different frameworks break the PDLC into six or seven stages, but they all describe the same journey:
- Generate and refine ideas.
- Design and prototype solutions.
- Develop and test them under representative lab or pilot conditions.
- Bring them to market.
- Learn from performance and iterate.
Historically, organizations treated the PDLC as a linear checklist: finish one stage, pass a gate, move on. Today, leading teams in the pharmaceutical, biotechnology, food & beverage, chemicals, and advanced materials industries view it as a continuous feedback system. Data from experiments, pilot runs, and customer usage flows back into earlier stages of the cycle. This new information allows teams to reshape approaches, requirements, formulations, processes, and parameters.
In materials-based industries, where each iteration is costly and high-stakes, a modern product development lifecycle gives R&D, production, quality, and commercial teams a shared structure and language. It enables faster, evidence-based decisions and a more reproducible and adaptable innovation pipeline, especially when development data is easier to access and apply. It also plays an important role in new product introduction, where teams must establish evidence for materials and processes that have never been run at scale.
Benefits of the Product Development Life Cycle
The PDLC offers many benefits, including:
1. Faster Time to Market
Clear stages and defined decision gates reduce ambiguity and rework. When teams see what “ready” looks like at each step, weak projects are shelved earlier, and promising ones move faster from lab to launch.
2. Improved Collaboration and Knowledge Sharing
When everyone works from the same lifecycle model, handoffs are smoother, especially when R&D, process, quality, and commercial development teams have the same access to databases of experimental results, formulations, and process parameters. Knowledge sharing helps your teams understand when they need to lean in, and ensures that decisions are documented in a way that others can reuse.
3. Higher Product Quality
A lifecycle perspective brings earlier visibility into the factors that influence product performance. Issues related to formulation, materials selection, or process conditions can be detected sooner, which reduces late-stage rework and strengthens overall product reliability.
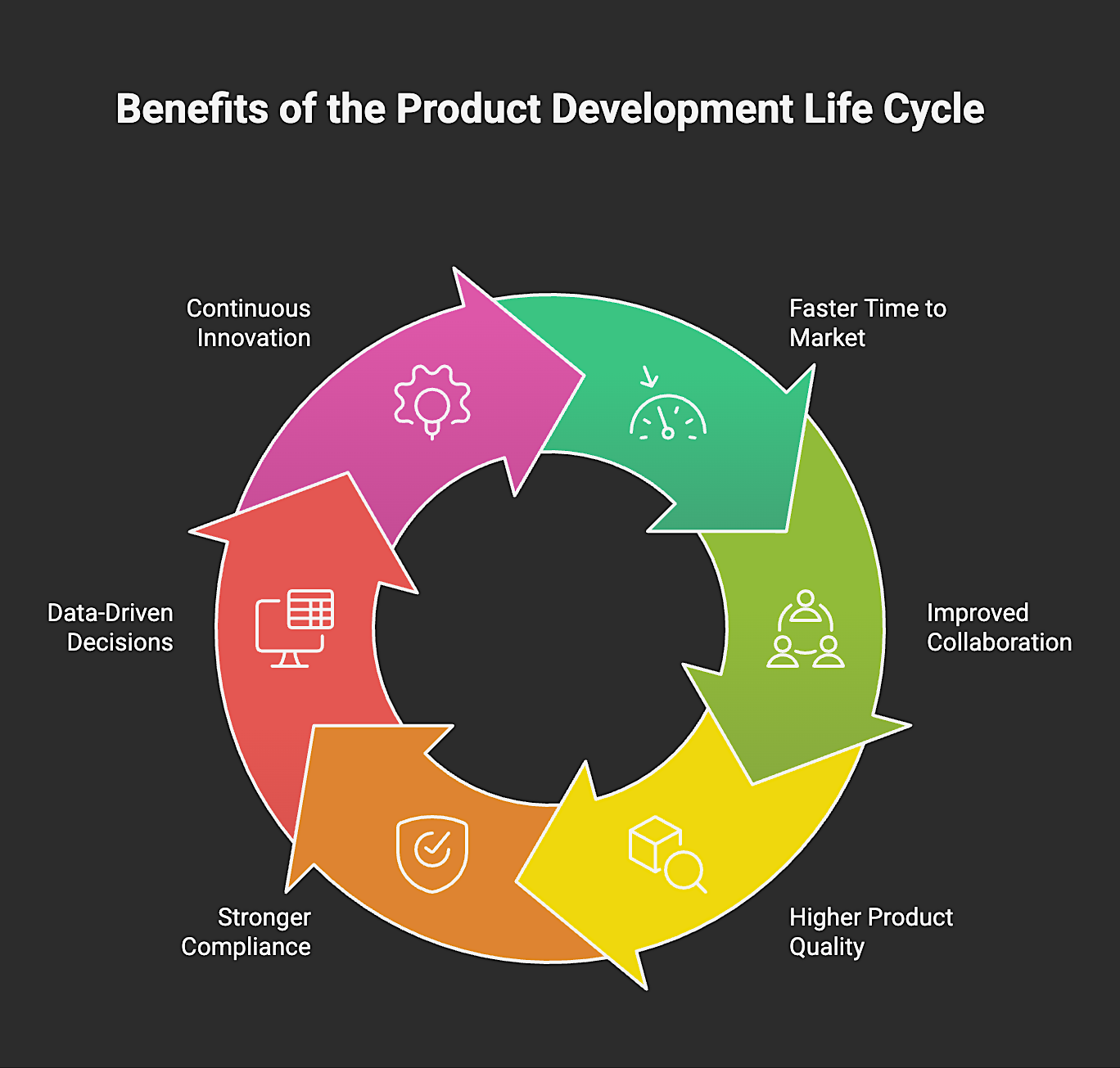
4. Stronger Compliance Posture
The product development lifecycle supports traceability and documentation practices that make it easier to meet evolving regulatory expectations. These include logging safety considerations, regulatory exposure, and alignment with continuous process verification in highly regulated environments.
5. Smarter, Data-driven Decisions
Centralizing materials, experiments, processes, and performance data in one environment enables teams to ask better questions (such as which parameters drive yield, which patterns usually fail at scale-up, etc.), and decisions are guided by evidence rather than memory.
6. Continuous Innovation
Learning doesn’t stop at launch, and a strong PDLC closes the loop. Field and manufacturing data inform roadmap choices and improve next-generation products without reinventing the process every time.
The 7 Stages of the Product Development Life Cycle
Here are seven practical stages to describe the PDLC. They’re shown in sequence for clarity, but teams move between them as new data emerges. At each stage, the focus is on the decision that needs to be made and the necessary evidence to support it.
Stage 1: Ideation and Concept Development
Stage 1 is about choosing the right problems to solve. Scan market, technology, and regulatory trends relevant to your materials or application space, then translate them into opportunity statements for materials-based products or formulations.
In practice, that means combining inputs from R&D, process engineering, commercial, and sustainability teams to frame concepts that are technically plausible and aligned with strategy.
Organizations increasingly use data to guide this step. They consolidate past experiments, material performance data, and relevant operational insights to focus on concepts with a realistic path to scale. A centralized, searchable knowledge base, such as a materials informatics platform, helps capture prior learnings and feed stronger concepts for the subsequent stages.
Stage 2: Research and Feasibility Analysis
In Stage 2, promising opportunities become concrete product concepts. Teams define the product’s intended users or applications, what problem it solves, and what performance, cost, and sustainability targets it must meet. Then, those hypotheses are stress-tested against rapidly shifting technical, market, and regulatory realities.
With robust materials informatics tools, you can run AI-driven feasibility testing and outcome forecasting when adequate historical data exists and models are validated. The insights gained by combining historical datasets from lab, pilot, and manufacturing processes will inform better decision-making. Teams can explore how changes in materials or parameters could influence performance and manufacturability before committing to physical experiments.
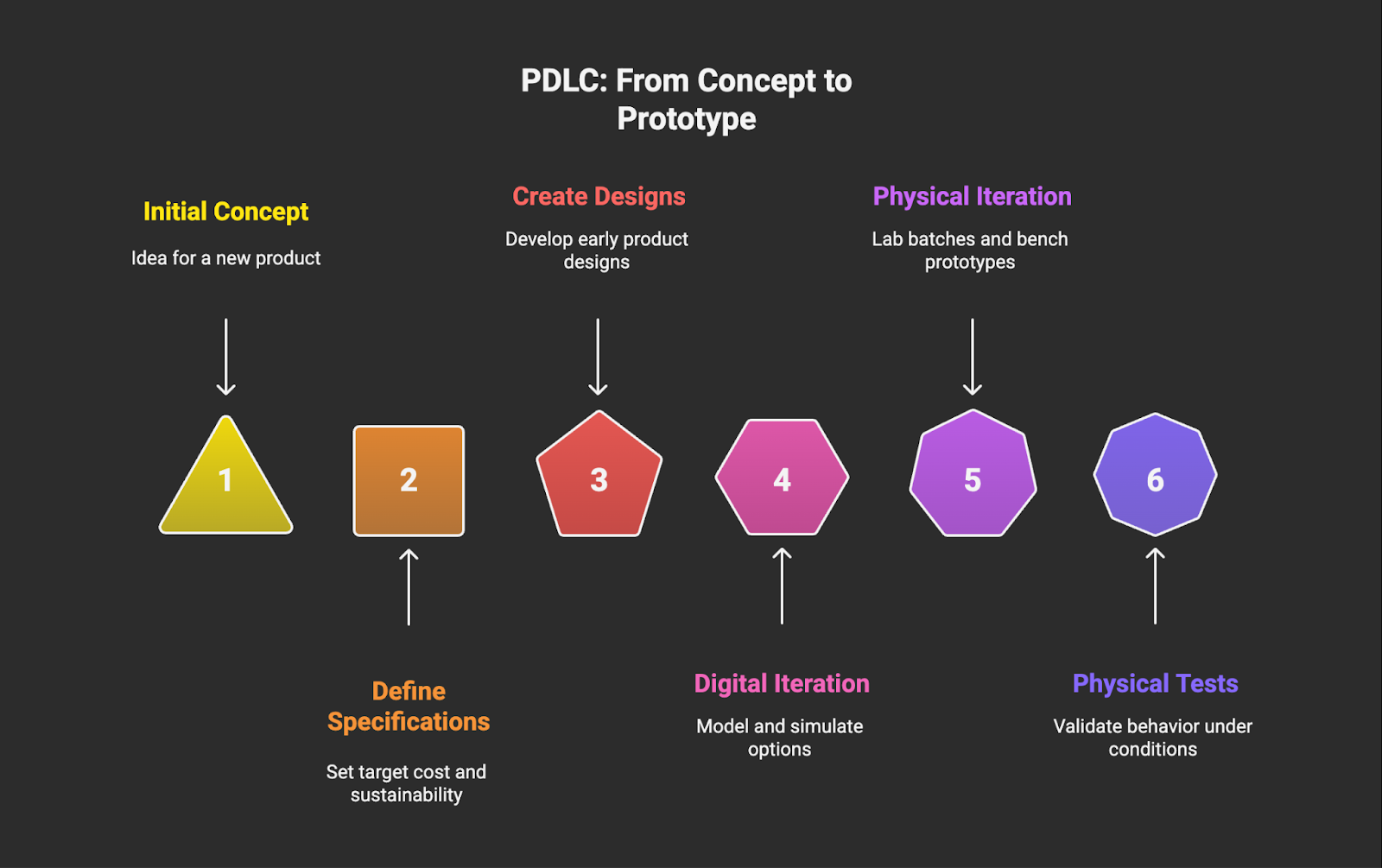
Stage 3: Design and Prototyping
Stage 3 is where concepts are transformed into something that can actually be tested. Define target specifications such as cost and sustainability, then create early designs or trial formulations that represent the intended product. It typically involves translating requirements into process parameters that can be evaluated efficiently in the lab.
For product development life cycles in materials science, design and prototyping often involve combining digital and physical iterations: modeling and simulation to narrow down options, followed by lab batches, bench prototypes, and physical tests to validate behavior under representative conditions.
Stage 4: Development and Testing
In Stage 4, the focus is on turning promising formulations or prototypes into well-characterized, robust products. R&D teams run structured experiments to refine compositions and understand how parameters affect relevant variables. In regulated sectors like pharma and food & beverage, this often includes stability studies and predefined design-of-experiments (DoE) to map the design space before formal validation.
Modern “lab of the future” initiatives show how digital tools transform this stage: integrated software systems and advanced AI-driven analytics reduce errors and manual handoffs, while accelerating testing cycles and maintaining data integrity and traceability.
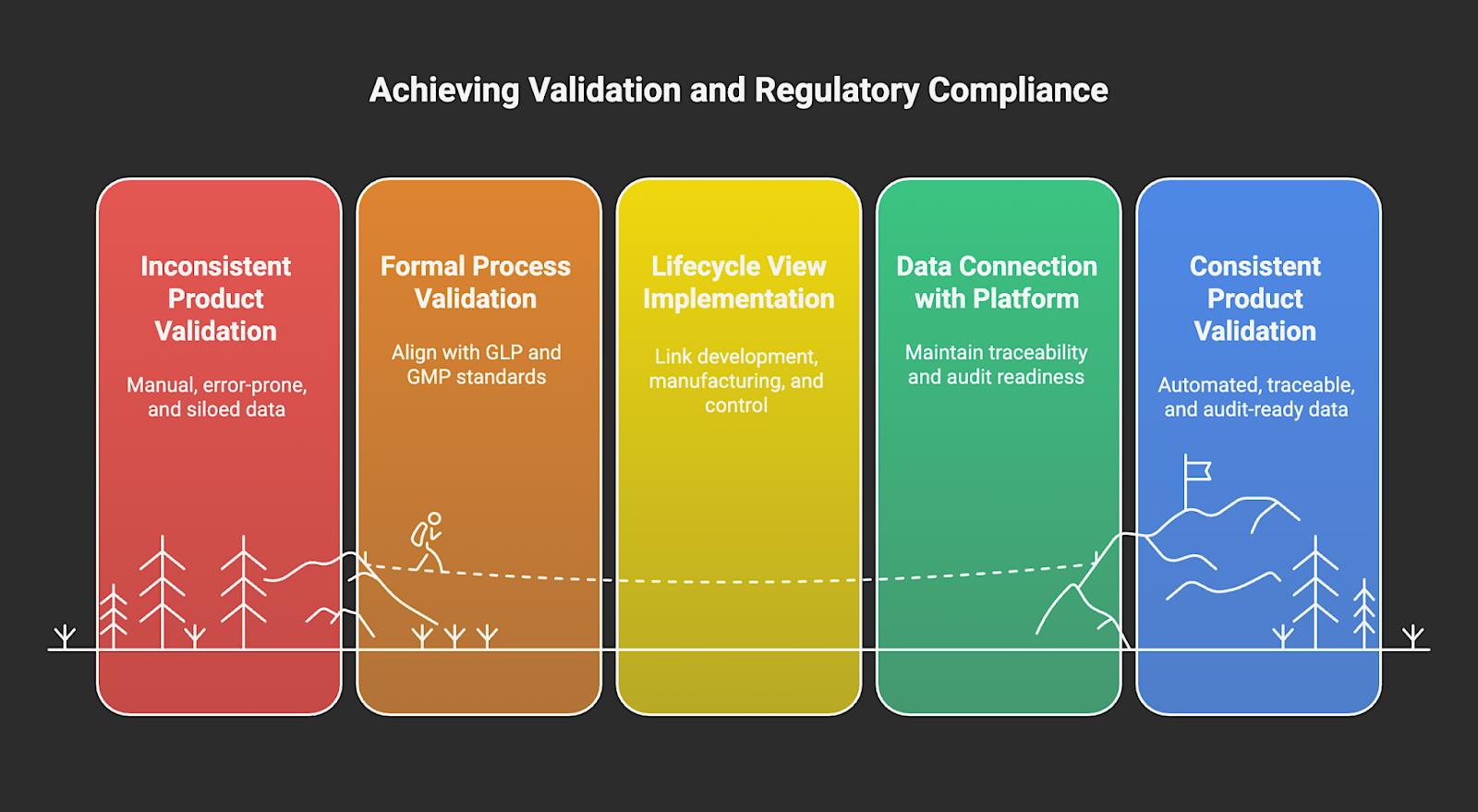
Stage 5: Validation and Regulatory Compliance
This step is where you prove that the product and process consistently meet defined requirements and regulatory standards. In materials-based and life-science industries, that typically means formal process and method validation with documentation aligned with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP).
Regulators now expect a lifecycle view of validation rather than a one-off event. They require linking product and process development, commercial manufacturing, and ongoing process control verification.
Platforms like MaterialsZone can act as the evidence backbone at this stage by connecting all relevant data in a way that enables teams to maintain traceability and audit readiness without drowning in manual paperwork.
Stage 6: Commercialization and Launch
In Stage 6, the work shifts from being “ready” on paper to executing in the market. With materials-based and life-science products, launch is a coordinated effort across technical, commercial, and supply chain teams.
Key focus areas typically include:
- Market and access readiness: Align claims, labelling, and documentation long before approval and launch. In sectors like pharma and medical that require formal market access activities, teams also prepare the corresponding regulatory and commercial plans.
- Supply and operations readiness: Confirm qualified suppliers, capacity, logistics, and service models for new materials, ingredients, or devices (often with long customer qualification cycles).
- Go-to-market alignment: Equip sales, technical service, customer success, and marketing with clear value propositions and success metrics, so they can target the right segments.
Stage 7: Post-Launch Evaluation and Continuous Improvement
The PDLC does not end with the launch but with the start of the next learning cycle. In Stage 7, monitor how the product performs in the market and in production across:
- Adoption
- Retention
- Quality trends
- Yields
- Costs
- Sustainability or regulatory signals
Many modern frameworks explicitly treat post-launch analysis and continuous improvement as a separate stage that’s focused on tracking KPIs and feeding insights from user feedback into the roadmap.
For materials-based industries, this stage is where lifecycle thinking materializes. Guidance like ICH Q10 for pharmaceuticals frames quality and improvement across the entire product lifecycle, linking development, commercial manufacturing, and post-market activities.
Materials informatics platforms support this by unifying external, laboratory, plant, and field data into a single environment. This way, teams can identify issues early and design the next round of experiments or product variants based on concrete evidence.
Strengthen Your Product Development Life Cycle with MaterialsZone
A modern PDLC is a shared, data-driven approach to rapidly innovate in complex, regulated environments without increasing business risk. On its own, this process is effective, but it’s not enough in modern R&D environments.
When you connect the product development lifecycle to a comprehensive materials informatics platform like MaterialsZone, you give every stage a stronger foundation. You get richer historical context at ideation, smarter feasibility checks, faster optimization, smoother validation, and a tighter post-launch feedback loop. The result is a product development engine that strengthens its insights over time.
Request a MaterialsZone demo to discover how it strengthens each stage of your product development life cycle.


.png)

